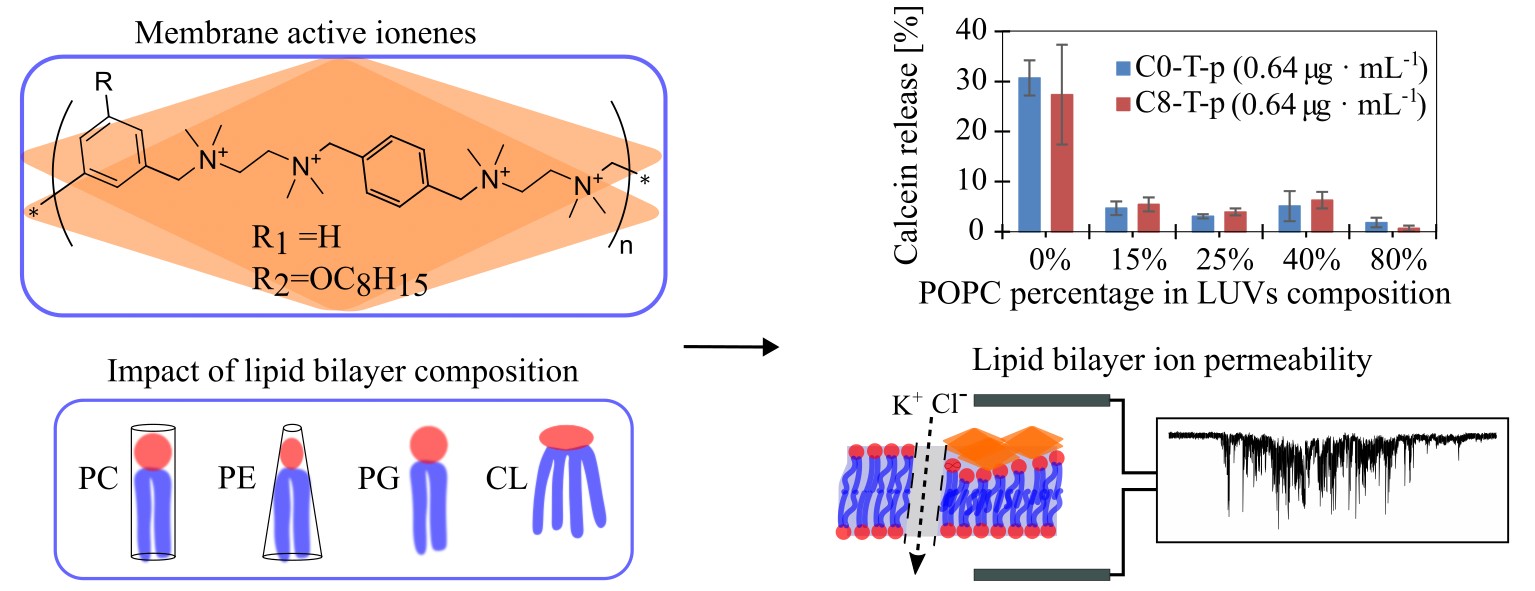
Cell wall disruption of the lipid bilayer, mediated by natural and synthetic macromolecules, is widely exploited in applications such as drug and gene delivery and antimicrobial therapies. This project aims to research the mechanisms by which specific antimicrobial amphiphilic polymers interact with cell membranes, with a focus on how these polymers disrupt lipid bilayers. To this end, we will investigate the membrane-disrupting properties of amphiphilic polymers derived from co-polymeric derivatives of linear polyethylenimine (LPEI-d) and co-polymers of maleic anhydride (PMA-d). By examining these polymers, we seek to understand their structure-activity relationships, especially regarding their behavior within artificial cell membranes, and to optimize their molecular design for maximum effectiveness against model organisms such as Escherichia coli and Staphylococcus aureus.
Building on this knowledge, we will test the hypothesis that amphiphilic polymers with switchable antimicrobial properties can be constructed and activated by specific stimuli relevant to physiological conditions, such as pH, temperature, or redox potential. We will fabricate and evaluate these macromolecules, aiming to develop polymers with controllable antimicrobial activity that can be turned on or off under various conditions.
Finally, this project will investigate whether the antimicrobial polymers studied can be further modified to achieve substantial cell membrane disruption against Mycobacteria, a target of particular importance given the urgent need for new methods to combat antibiotic-resistant Mycobacterium tuberculosis. This objective is especially significant in light of the challenges posed by tuberculosis, which continues to spread despite advancements in medicine, partly due to factors like inadequate control over antibiotic use in developing regions.
The research objectives will be met through the synthesis and analysis of libraries of novel polymers, utilizing RAFT (Reversible Addition-Fragmentation Chain Transfer) and ROP (Ring Opening Polymerization) techniques. The resulting macromolecular structures will undergo detailed structural analysis to identify key structure-activity relationships. Standard characterization techniques such as NMR, MALDI-TOF, and GPC will be employed, alongside micellar characterization methods like dynamic light scattering (DLS), zeta potential measurements, TEM, Langmuir–Blodgett balance, and quartz crystal microbalance (QCM) to investigate the cell membrane disruption mechanisms. Additionally, bioactivity testing will be conducted against model membrane structures, E. coli, S. aureus, and Mycobacterium.
Understanding the mechanisms of action of antimicrobial amphiphilic polymers will offer valuable insights for designing future antimicrobial materials and novel active pharmaceutical ingredients. Beyond simple bactericidal activity, these materials may also facilitate membrane transport for other molecules such as genes or proteins. The project’s focus on polymer activity against Mycobacterium holds particular promise, as the antibiotic resistance associated with tuberculosis-related bacteria presents a critical global health challenge. This project aims to contribute important knowledge toward combating Mycobacterium and addressing the spread of antibiotic resistance.