by
Kurzyna, J. M.; Kopiasz, R. J.; Paul, M.; Flont, M.; Baranowska, P.; Mierzejewska, J.; Drężek, K.; Tomaszewski, W.; Jastrzębska, E.; Jańczewski, D.
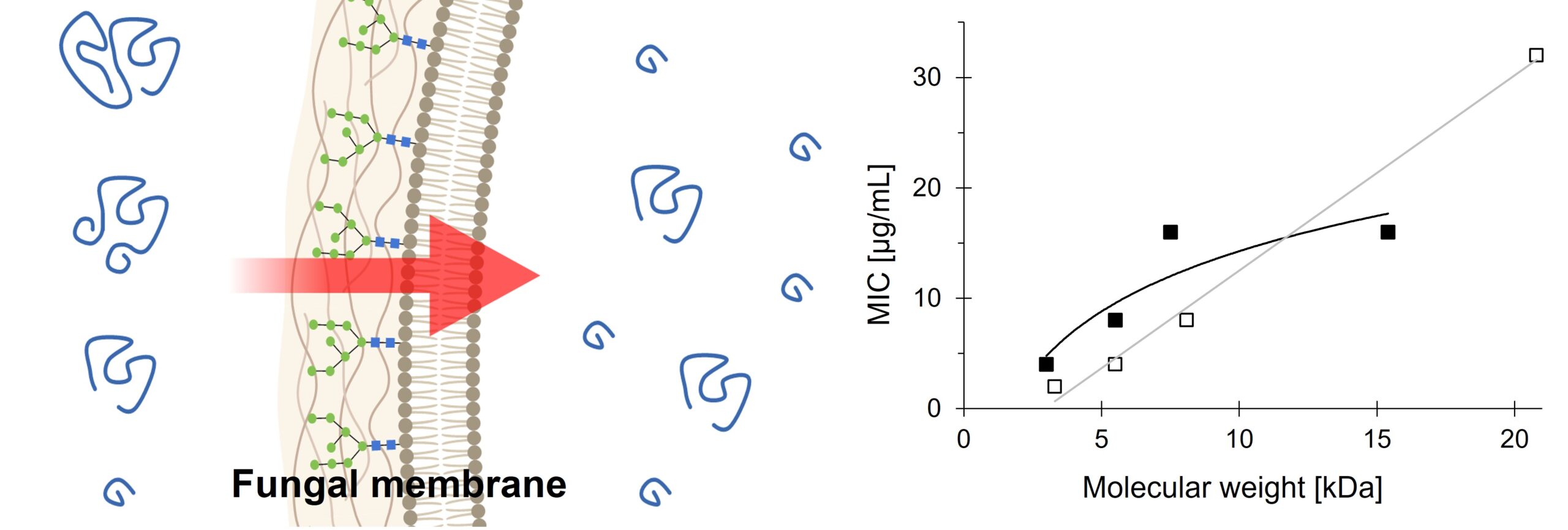
Numerous synthetic polymers, imitating natural antimicrobial peptides, have demonstrated potent antimicrobial activity, positioning them as potential candidates for new antimicrobial drugs. However, the high activity of these molecules often comes at the cost of elevated toxicity against eukaryotic organisms. In this study, a series of cationic ionenes with varying molecular weights to assess the influence of polymer chain length on ionene activity is investigated. To enhance polymer antimicrobial activity and limit toxicity a PEG side chain is introduced into the repeating unit. The resulting molecules consistently exhibited high activity against three model organisms: E. coli, S. aureus and C. albicans. The incorporation of side PEG chain improves antifungal properties and biocompatibility, regardless of molecular weight. The most important finding of this work is that the reduction of polymer molecular mass led to increased antifungal activity and reduced cytotoxicity against HMF and MRC-5 cell lines simultaneously. As a result, the best-performing molecules reported herein displayed minimal inhibitory concentrations (MIC) as low as 2 and 0.0625 μg·mL-1 for C. albicans and C. tropicalis respectively, demonstrating exceptional selectivity. It is plausible that some of described herein molecules can serve as potential lead candidates for new antifungal drugs.